
ZYRTEC®
Children’s ZYRTEC® delivers effective, 24-hour allergy relief with convenient once-daily dosing and kid-friendly forms and flavors. For ages 2+.
Recommend effective relief for kids’ worst allergy symptoms: sneezing; runny nose; itchy, watery eyes; and itchy throat or nose.
chewable-tout-bkg2x.jpg

Children’s ZYRTEC® Chewables
- Relieve the worst allergy symptoms,
indoors and out* - Dye free
- Consistently powerful, 24-hour relief
*Relieves sneezing; runny nose; itchy, watery eyes; and itching of the nose or throat.
-
Dosing for Children’s ZYRTEC®
-
Children’s ZYRTEC® Allergy Syrup Dosage
Effective allergy relief in a sugar-free, dye-free syrup formulation in kid-friendly grape and bubble gum flavors

Active ingredient: Cetirizine HCl 5 mg (in each 5 mL) Age Dosage Adults and children 6 years and over 5 mL or 10 mL once daily depending on severity of symptoms; do not take more than 10 mL in 24 hours Adults 65 years and over 5 mL once daily; do not take more than 5 mL in 24 hours Children 2 to under 6 years of age 2.5 mL once daily; if needed, dose can be increased to a maximum of 5 mL once daily or 2.5 mL every 12 hours. Do not give more than 5 mL in 24 hours Children under 2 years of age Ask a doctor Consumers with liver or kidney disease Ask a doctor mL = milliliter
group_1513x.png
 Share Image:0
Share Image:0Children’s ZYRTEC® has no juice warning on the label, unlike Allegra®
Children’s ZYRTEC® Chewables Dosage
Effective allergy relief in dye-free, great-tasting chewables

Directions: May be taken with or without water. Chew or crush tablets completely before swallowing.
Active ingredient: Cetirizine HCl 2.5 mg or 10 mg (in each chewable tablet)Age Dosing:
CHILDREN’S ZYRTEC® CHEWABLES 2.5 MGDosing:
CHILDREN’S ZYRTEC® CHEWABLES 10 MGChildren 2 to under 6 years of age Chew and swallow 1 tablet (2.5 mg) once daily; if needed, dose can be increased to a maximum of 2 tablets (5 mg) once daily or 1 tablet (2.5 mg) every 12 hours. Do not give more than 2 tablets (5 mg) in 24 hours. Ask a doctor Adults and children 6 years and over Chew and swallow 2 tablets (5 mg) or 4 tablets (10 mg) once daily depending upon severity of symptoms; do not take more than 4 tablets (10 mg) in 24 hours. Chew and swallow 1 tablet (10 mg) once daily; do not take more than 1 tablet (10 mg) in 24 hours. A 5 mg product may be appropriate for less severe symptoms. Adults 65 years and over Chew and swallow 2 tablets (5 mg) once daily; do not take more than 2 tablets (5 mg) in 24 hours Ask a doctor Children under 2 years of age Ask a doctor Ask a doctor Consumers with liver or kidney disease Ask a doctor Ask a doctor mg = milligram
Children’s ZYRTEC® Dissolve Tabs Dosage
Melt-away tablets for 24-hour relief from kids’ indoor and outdoor allergy symptoms

Active ingredient: Cetirizine HCl 10 mg (in each tablet) Directions: Tablet melts in mouth. Can be taken with or without water. Age Dosage Adults and children 6 years and over One 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms Adults 65 years and over Ask a doctor Children under 6 years of age Ask a doctor Consumers with liver or kidney disease Ask a doctor mg = milligram
-
-
Pediatric Clinical Data
-
EFFICACY DATA
Children’s ZYRTEC® makes a significant impact on allergy symptoms1*†
Powerful symptom response seen in pediatric patients with seasonal allergic rhinitis (SAR)1
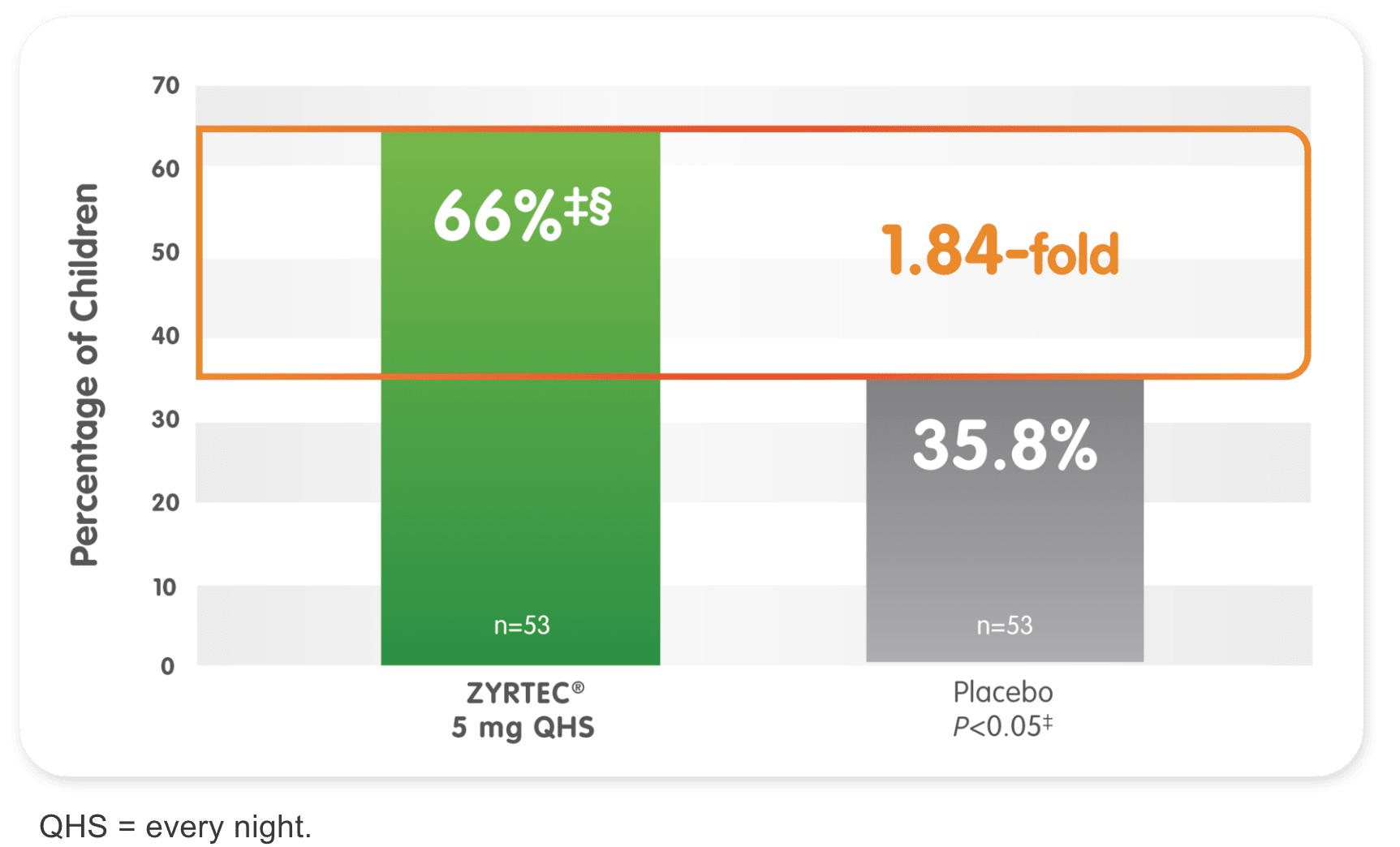
Efficacy in ages 2 to 6: Children’s ZYRTEC® 5 mg
66% of children demonstrated ≥50% improvement from baseline SAR symptoms over 2 weeks1
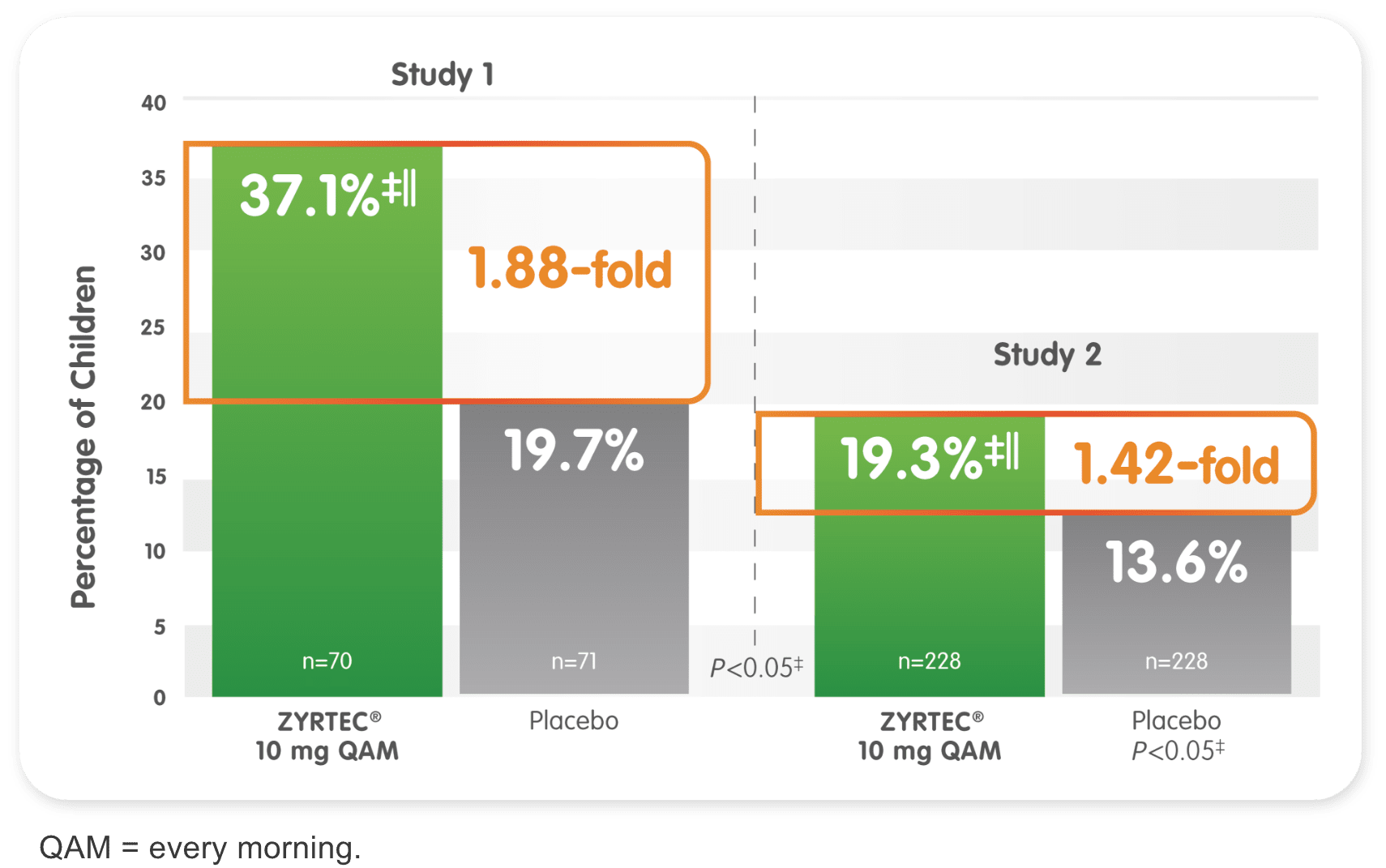
Efficacy in ages 6 to 11: Children’s ZYRTEC® 10 mg
Studies show 37% and 19% of children demonstrated ≥50% improvement from baseline SAR symptoms over 2 weeks1
*Randomized, placebo-controlled study of children aged 2 to 6 years with SAR during a 2-week period. Primary endpoint was the change in total symptom score complex from baseline.
†Randomized, placebo-controlled studies of children aged 6 to 11 years with SAR during a 2-week period. Primary endpoint was the change in total symptom score complex from baseline.
‡Significant vs placebo.
§ZYRTEC® 5 mg vs placebo ratio at ≥50% reduction is 1.84-fold.
||ZYRTEC® 10 mg vs placebo ratio at ≥50% reduction is 1.88-fold in Study 1 and 1.42-fold in Study 2.CONSISTENCY DATA
ZYRTEC® provides proven, consistent symptom relief
Children’s symptoms improved consistently over a 2-week period*
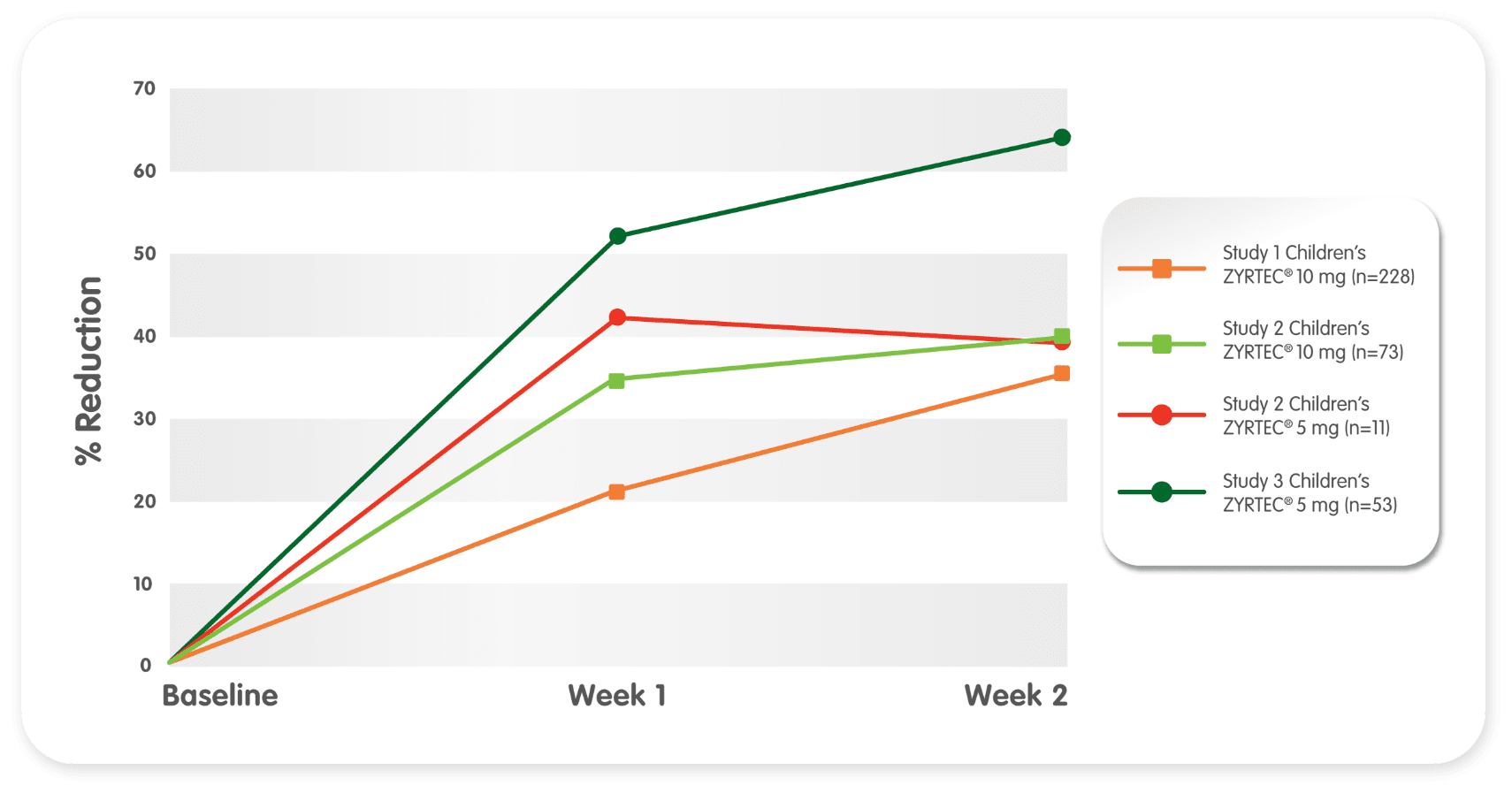
Reduction from baseline in total symptom severity complex (TSSC)2†
- 37% and 40% reduction observed after 2 weeks of treatment in the 10-mg group
- 63% and 40% reduction observed after 2 weeks of treatment in the 5-mg group
*All studies are multicenter, randomized, placebo-controlled, double-blind in children ages 2 to 11 years with previous diagnosis of SAR, and adequate minimum duration (2 weeks).
†Adjusted mean change relative to baseline.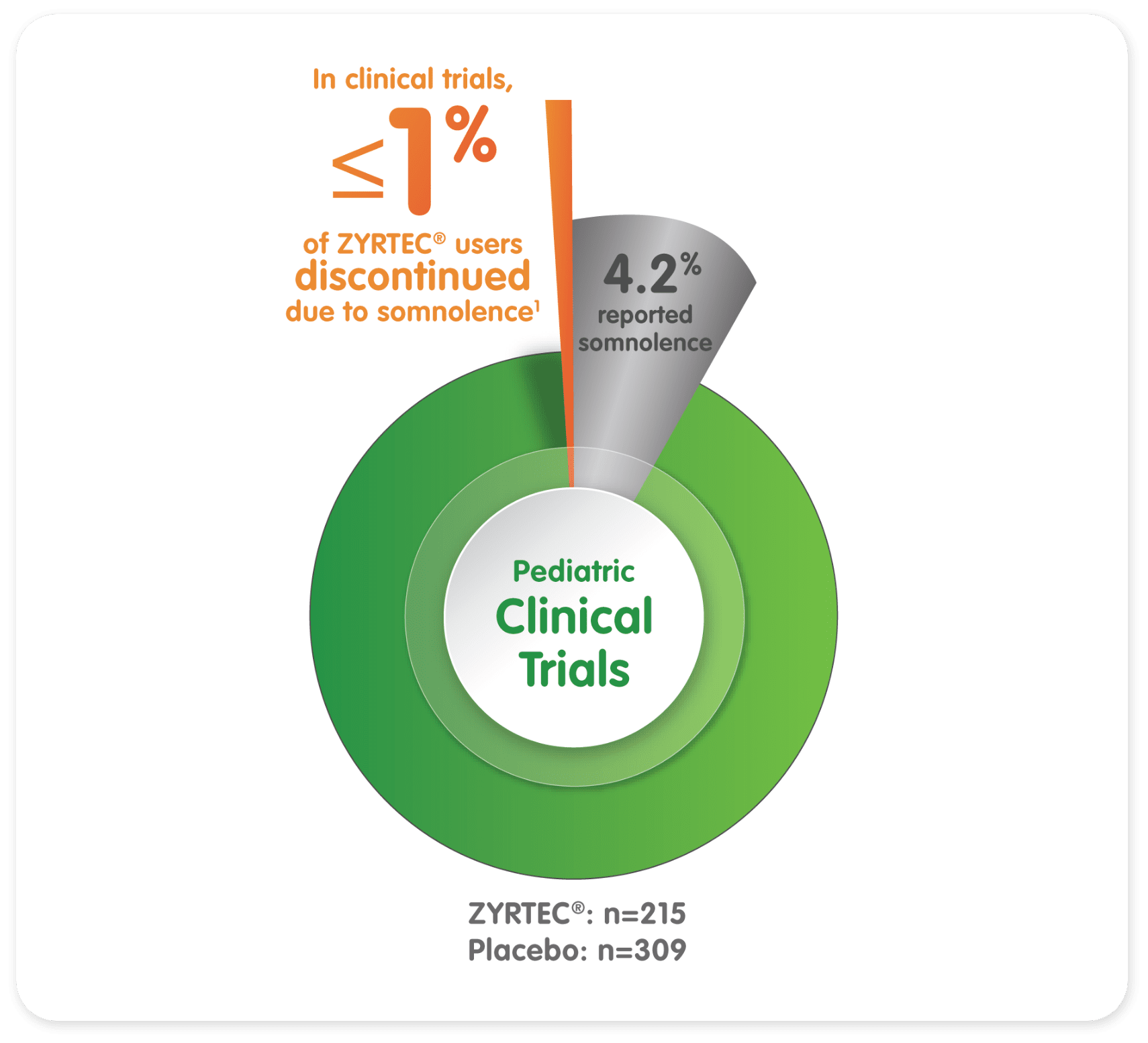
TOLERABILITY DATA
ZYRTEC® is generally well tolerated by children1
In studies of children ages 6 to 11 years:
- 4.2% of patients taking ZYRTEC® 10 mg reported somnolence (n=215) vs 1.3% with placebo (n=309)1
- ≤ 1% of ZYRTEC® patients discontinued due to somnolence1
References: 1. Data on file. Johnson & Johnson Consumer Inc., McNeil Consumer Health Division. 2. Urdaneta ER, Przygoda PA, Lin CL, et al. Regular use of cetirizine demonstrates consistent relief from symptoms of seasonal allergic rhinitis in children. J Allergy Clin Immunol. 2011;127(suppl 2):AB 202.
-
bottom-chewable-tout-bkg2x.jpg

See how Children’s
ZYRTEC® compares
View chart comparing pediatric OTC oral antihistamines.
Want to explore Children’s ZYRTEC® products for your pediatric patients?
Effective relief when kids need it most
When their allergies act up, recommend BENADRYL®
Monthly Pediatric BundleBox
Discover what’s inside



All Fields required, unless otherwise indicated
Will be used as your user name
By submitting your information above, you agree that the information you provide will be governed by our site's Privacy Policy.